Sunlight Harvesting [~43K] (Painting by Glynn
Gorick)
![]()
Gaseous Composition of the Atmosphere [~41K]
[~125K] Replenishment of NO3 and NH4 by N2-Fixation
![]()
[~325K] Global Distribution of Trichodesmium
![]()

[~350K] Trichodesmium bloom in the Capricorn Channel, nr.
Australia (photo by NASA)
Phytoplankton are the ocean's equivalent of plants on land - the organisms which harvest the sun's light and use it to grow with. But in contrast to the plants we know on land: trees, grasses, shrubs and the like, most plants in the ocean are just little dots. In fact they are so small that those dots are mostly invisible to the naked eye! It was Antonie van Leeuwenhoek, a Dutch lens-grinder, who in 1676 was the first person on Earth to take samples of seawater and look at them under a powerful magnifying lens. He saw an incredible array of little creatures - the until then invisible plants and animals of the sea.
Although much smaller than their compatriots on land, the miniscule ocean plants are not thereby spared the need to obtain essential nutrients in order to grow and multiply. But which of these nutrients (carbon, nitrogen, phosphorus, sulphur, ..) is most important in controlling growth, or is the availability of light more importan?
When scientists see what they can do to coax phytoplankton into growing faster, they typically get the best results by adding nitrogen in the form of nitrate (NO3) or ammonium (NH4). This is because this nitrogen is the most scarce of all nutrients in surface waters, relative to the amounts needed by phytoplankton. So a hypothetical farmer of the sea would fertilise his waters with nitrogen salts if he wanted to maximise his plant crop, as real farmers indeed do on land.
What is nitrogen fixation?
Nitrogen fixation is no more nor no less than the use of di-nitrogen (N2) as the source of nitrogen atoms for growth. But why is the use of N2 to get nitrogen given a special name to distinguish it from the use of NO3 or NH4? To answer this we need to briefly consider the nature of N2.
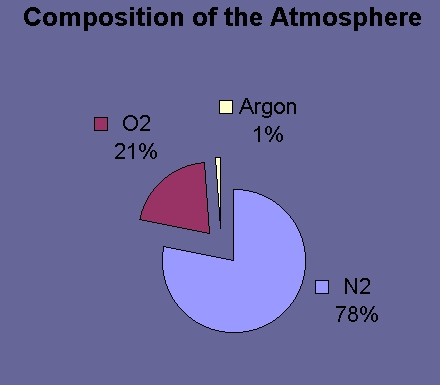
The mouthful of air you are currently breathing in is made up of about 78% di-nitrogen, 21% oxygen and 1% argon. The Earth's atmosphere is mostly N2. N2 is also very abundant in seawater, because some of the enormous amount in the atmosphere diffuses across the sea surface and into the water.
But yet, somewhat incredibly, most phytoplankon in the ocean are unable to use this abundantly available form of nitrogen. This is because the two atoms in the N2 molecule are very firmly bound together, with lots of energy being required to break them apart. Nitrogen in the oceans that occurs as the dissolved ions nitrate (NO3) and ammonium (NH4), on the other hand, is much more convenient to use because less energy expenditure is required. It is as if the N atoms in an N2 molecule are held together using chains, whereas linked into NO3 and NH4 molecules using only string. (another analogy)
So N2 is super-abundant but difficult to use, whereas NO3 and NH4 are easy to use but inconveniently occasionally run out. The inert nature of N2 molecules leads to them only being used as a last resort, hence the special name of nitrogen-fixation for N2-usage.
Why is nitrogen-fixation important?
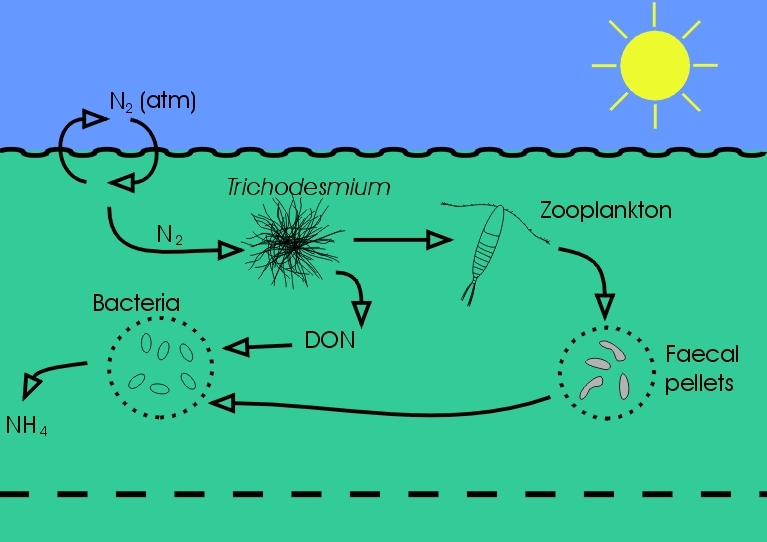
The real magic of nitrogen-fixation is not just that it allows some plants to continue growing even when nitrate and ammonium have run out, but also that it replenishes those other forms of nitrogen, fertilising the ocean so that the NO3- and NH4-using phytoplankton can grow once more. Returning to the farming analogy, nitrogen-fixation is a free and natural source of nitrogen fertiliser. Nitrogen-fixers act as a conduit of the inert N2 to the more readily usable forms of NO3 and NH4.
The rate of supply of this natural nitrogen fertiliser can be so important that in many regions of the sea it controls how many phytoplankton grow there. This in turn controls how many fish, whales and other animals can live there, since phytoplankton are the base of marine food chains. It also determines how much carbon dioxide that part of the ocean `sucks down' from the atmosphere.
Which phytoplankton can fix nitrogen?
The few members of the phytoplankton which can use N2 are exclusively members of an ancient division of life, the cyanobacteria. Cyanobacteria first evolved more than 2 billion years ago, when the Earth's atmosphere was rich in carbon dioxide but devoid of oxygen. This type of organism is at least half as old as the Earth itself.
These descendants of survivors from an oxygen-less ocean still today show signs of their ancient lineage. The enzyme they use to fix nitrogen is deactivated by oxygen. Because the water they live in today is always highly oxygenated, the cyanobacteria have had to create special conditions inside their cells in order to isolate the enzyme from the oxygen everywhere outside.
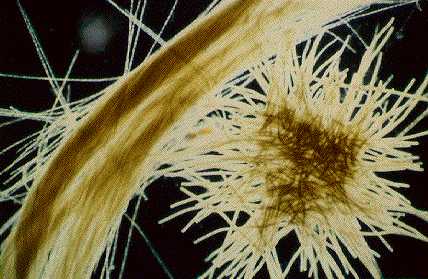
Most phytoplankton are microscopic isolated dots, each one floating alone. But In some species the individual cells congregate together to form chains or spirals or other shapes, thus passing their short lives in a more sociable fashion.
In the main nitrogen-fixing species in the ocean, Trichodesmium, the individual cells often join together in a line to form a filament of about 100 cells. These filaments themselves also join together, in a second level of organisation, this time to form bundles of filaments known as colonies, each one being made up of 100 or so filaments, i.e. ~10,000 cells in total. This lifestyle may make it easier to protect the nitrogen-fixing enzyme from oxygen, if many cells are adjacent to other cells rather than always adjacent to water on all sides.
Some other nitrogen-fixing cyanobacteria found in the oceans and seas are: Richelia intracellularis as a symbiont within Rhizosolenia or Hemialus, Nodularia spumigena and Aphanizimenon, with the latter two restricted to fresh or coastal waters such as the Baltic Sea. Some smaller and less conspicuous nitrogen-fixers may be out there still awaiting discovery.
Where do nitrogen-fixers live in the ocean?
The distribution of N2-fixers is patchy in both time and space, but in the open ocean they are restricted to low latitude waters, within about 30 or 40 degrees of the equator, and can occur at any time of year. The Sargasso Sea, the Caribbean, the ocean west of west Africa, the east and north coasts of Australia, the seas around Fiji, the Red Sea and the Arabian Sea are some areas of the highest concentrations of Trichodesmium. In the Baltic Sea nitrogen-fixers occur everywhere except for the northern-most part (Bothnian Bay), and they occur mainly in the summer.
Being photosynthetic organisms nitrogen-fixing phytoplankton are restricted to the sunlit zone, that is the top 100 m or so, below which insufficient sunlight penetrates. The question of why nitrogen-fixers are constrained to low latitudes in the open ocean, and why they flourish in some low latitude areas but not others, is an exciting area of current investigation.